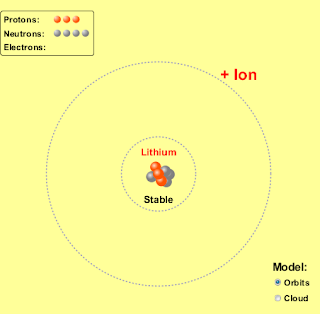
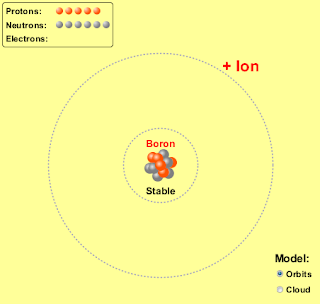
1. Run the Build an Atom simulation http://phet.colorado.edu/en/simulation/build-an-atom and build a neutral lithium atom and a neutral boron atom. Take a picture, or a screen shot, of these two atoms and place them on your blog. List the number of protons, neutrons and electrons for each. Also look up and post the density for each of the elements on your blog.
| Element | Picture | Protons | Neutrons | Density @ 293 K (g/cm3) |
| Lithium (Li) | | 3 | 4 | .53 |
| Boron (B) | | 5 | 6 | 2.34 |
2. Define density and the equation for density and post on your blog.
Density is a physical property of matter. It is the measure of the relative “heaviness” of objects with a constant volume. It is measured in g/mL. This could mean how “packed” the material appears to be.
Density =Mass/Volume
3. Run the Density simulation http://phet.colorado.edu/en/simulation/density and complete one (your choice) of the prepared Teaching Ideas and post your results on your blog.
Student Guide for Density Simulation
(note: “sink” means stays on the bottom)
Start:
Google “Phet Density sim”
Click on the first link
Experiment with choosing a material:
| Sinkers | Floaters | Density Given |
| Aluminum | | 2.7 kg/L |
| | Wood | .40 kg/L |
| | Styrofoam | .15 kg/L |
| | Ice | .92 kg/L |
| Brick | | 2.00 kg/L |
Try to get aluminum to float. Talk with your partner about this possibility- can you change the mass of the aluminum block without changing the volume of the aluminum block? No you cannot change the mass without changing the volume.
What do you and your partner notice about the density triangle at the bottom of the box? Talk about why you think the slider moves or not. Aluminum has the same density no matter what. The volume changes with the mass so it will always stay the same.
How does the density of aluminum (2.70 kg/L) help explain what you see? The density is at its highest which is why it sinks. For example, Styrofoam is .15 kg/L which is a lot less dense.
Frame: The aluminum will sink in the water because the density of the aluminum is 2.70 kg/L3 and the density of water is 1000 kg/L3. We have learned that the density needs to be below 1 kg/L to float in water.
Density = ---------- “mass over volume equals density”
In the “Blocks” box, click on Mystery:
Test the boxes in the water- just drag and drop!!!
When you have determined which ones sink and float, fill in the data table for each box.
| Sample | Beginning amount of water (A) | Ending amount of water (B) | Volume (L) (difference B-A) | Mass (kg) | Density (kg/L) | What is it most likely made of? (hint: use Show Table for help) |
| A | 100-L | 103.38 L | 3.38 | 65.14 | 19.27 | Gold |
| B | 100-L | 100.64 L | .64 | .64 | 1 | Water |
| C | 100-L | 104.08 L | 4.08 | 4.08 | 1 | Water |
| D | 100-L | 103.10 L | 3.08 | 3.10 | .99 | Water |
| E | 100-L | 101.00 L | 1.00 | 3.53 | 3.53 | Diamond |
Look closely at green box C and red box D and discuss your observations. The two are very closely related in density and I have concluded that they must both be water.
List three observations you made while comparing the two boxes.
| 1st observation | 2nd observation | 3rd observation |
| Similar size | Both float with a slight difference | Don’t have the same ending amount of water. |
Dear Students,
I am back to building a boat. I want to build the best boat ever!! My partner says I cannot put a refrigerator and a television in my boat because that would make it too heavy-and the boat might sink. Then we would be swimming with the sharks!!!!
What would you advise me to tell my friend? Is she right or wrong? Be sure to give me some evidence based on what you learned from the boxes or other places in this activity.
Signed, your teacher
I would say that there is a possibility if there is a large enough surface area and buoyancy. When experimenting with the boxes, I found that if you have a box with a great buoyancy, it will hold another box above it if the circumstances are right.
4. Complete the Mystery Blocks activity on the Density simulation. Post on your blog the data you collected (mass, volume, and density) and the identification of the material and the known density.
| Sample | Beginning amount of water (A) | Ending amount of water (B) | Volume (L) (difference B-A) | Mass (kg) | Density (kg/L) | What is it most likely made of? (hint: use Show Table for help) |
| A | 100-L | 103.38 L | 3.38 | 65.14 | 19.27 | Gold |
| B | 100-L | 100.64 L | .64 | .64 | 1 | Water |
| C | 100-L | 104.08 L | 4.08 | 4.08 | 1 | Water |
| D | 100-L | 103.10 L | 3.08 | 3.10 | .99 | Water |
| E | 100-L | 101.00 L | 1.00 | 3.53 | 3.53 | Diamond |
5. Identify and post on your blog the Science Standards that could be met through these activities completed in Activity 5
Content Standard: Students in Wisconsin will understand that there are unifying themes: systems, order, organization, and interactions; evidence, models, and explanations; constancy, change, and measurement; evolution, equilibrium, and energy; form and function among scientific disciplines.
Content Standard: Students in Wisconsin will investigate questions using scientific methods and tools, revise their personal understanding to accommodate knowledge, and communicate these understandings to others.
Content Standard: Students in Wisconsin will demonstrate an understanding of the physical and chemical properties of matter, the forms and properties of energy, and the ways in which matter and energy interact.
Content Standard: Students in Wisconsin will demonstrate an understanding of the characteristics and structures of living things, the processes of life, and how living things interact with one another and their environment.
No comments:
Post a Comment